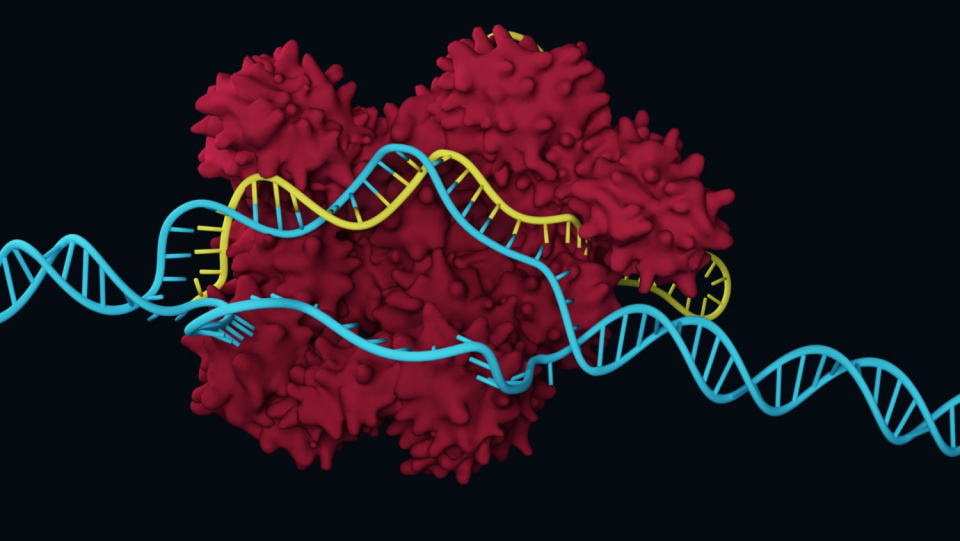
The "gene scissors" CRISPR/Cas9 celebrates its tenth birthday this year. Emanuelle Charpentier and Jennifer Doudna published their discovery in 2012 and were awarded the Nobel Prize in 2020. The originally bacterial cutting protein became a routine research tool in a very short time. It allows scientists to cut the genetic material quickly and easily in a targeted manner in all organisms.
The gene scissors also rekindled various hopes. For example that on functioning gene therapy. The idea behind it is captivatingly simple: many diseases are based on a gene error, a so -called mutation that makes the corresponding protein in good point of view. In classic gene therapy, the faulty gene is tried to replace with an intact, so that the cell is able to correctly produce the protein.
CRISPR/Cas9 is intended to enable even more precise intervention by repairing genetic defects directly on site, thus heralding a completely new era in medicine. "Good progress has been made in recent years," says Toni Cathomen, Director of the Institute for Transfusion Medicine and Gene Therapy at the University of Freiburg. But the closer you think you are to the goal, the clearer the existing challenges become.
Fresh blood as a result of a dislodged obstruction
The WHO lists 139 clinical studies in its genome genome editing registry genome editing registry. Basically, ex-vivo procedures in which patients are taken from cells and corrected in the laboratory are differentiated from in-vivo procedures in which gene correction takes place directly in the body. The possible applications are large and ranges from genetically ranged immune cells against cancer to therapy less frequently and often incurable hereditary diseases. Treatment against congenital blood cell anemia and β-thalassemia is most advanced, in which the body forms too little functional hemoglobin due to a gene error, which is responsible for the transport of oxygen in the blood.
The interim results of the first CRISPR-based gene therapy are positive: at the end of 2019, the first ß-thalassemia patient was treated at the University Hospital Regensburg. "The patient is fine, the therapy was successful," says Selim Corbacioglu, head of the Department of Pediatric Hematology, Oncology and Stem cell Transplantation there. The therapy also helped four other patients treated in Regensburg. You are no longer dependent on monthly blood transfusions and no longer have to hope for a suitable blood stem cell donor, because your body is now supplied with sufficient fetal hemoglobin.
This works with a trick: the gene for fetal hemoglobin is active during pregnancy, but is shut down some time after birth. With the help of the gene scissors, doctors cancel the blockade so that the body restores the fetal hemoglobin protein. The actual genetic defect is therefore not repaired, but a replacement gene is reactivated.
However, CRISPR gene therapy on cells outside the body (ex vivo) has the same serious side effects as conventional stem cell transplantation: after patients have had blood stem cells taken from them for editing in the laboratory, they need chemotherapy to destroy the defective, blood-forming cells. "This is almost inevitably associated with a loss of fertility," says Corbacioglu. In addition, it is not yet possible to adequately assess the late effects of gene therapy.
Body-wide gen therapy
The interim results of the study were published at a congress in autumn 2021: Two out of five patients reported an improvement in their vision. The judgement of the scientific community was mixed. Currently, other patients are being treated with a higher dose and also adolescents in whom the disease may not have progressed as far. Further results are expected in the autumn.
In 2021 the next application followed, which the specialist magazine "Science" for a "breakthrough of 2021" cited: 15 patients with the rare disease transthyretin-amyloidosis have now received crispr gener therapy by syringe-directly into the bloodstream. For them, the TTR protein is folded due to a mutation in the corresponding gene. It is formed in the liver and released into the blood, clumps and enriches itself primarily in the heart and nervous system, which leads to sensations, pain and muscle weakness. The gene scissors cut the TTR gene, so that the concentration of the protein drops by up to 93 percent.
Breaching is simpler.
In February, the companies involved in the study announced that the effects of one-time liver gen therapy continue after one year. However, it is still unclear whether the progression of the disease can be stopped or even reversed.
"What we're currently seeing across all studies is that cutting genes is much easier than repairing them," says Cathomen. CRISPR/Cas9 reliably cuts the DNA at the desired location, namely both strands of the DNA thread. However, the cell repairs the cut. It has two options for this: It usually reconnects the two loose ends of a DNA thread quite carelessly, i.e. errors occur. Errors in a gene usually lead to a non-functional protein – which leads to the goal in some gene therapies. "However, most genetic diseases can only be treated by correctly repairing the genetic defect," emphasizes Cathomen.
With the second, more precise repair mechanism of the cell, this can be achieved. However, it only works in dividing cells and is less active overall than the negligent variant. "The question is whether and how the exact repair can be activated," says Cathomen.
Cutting is also associated with a basic security risk, because now and then the gene scissors cut off. Scientists fear that this so-called off-tart activity could create cancer-causing mutations. "It won't work without any off-star effects," says Cathomen, "but not every bad cut is automatically problematic." Every person is increasing mutations in the course of their lives, that is a natural process. "The study participants are examined regularly, but we will only know how safe the technology is in a few years."
Is it simple to forgive mistakes?
Off-target effects may also have to be accepted: "It depends on the disease and the available therapies," explains Gerald Schwank from the University of Zurich. Although they cause many mutations. You accept that because the disease is life -threatening. "
Schwank is working on the second generation of CRISPR proteins, the so-called base and prime editors. With base editors, individual DNA building blocks can be exchanged. The most recent tool, the Prime editors, are more versatile because they can be used to edit larger sections of DNA. The intervention in the genome is less than with CRISPR/Cas9, since only one strand of the DNA thread is cut, it is very accurate and works even in dormant cells.
"There are estimates that 60 percent of human pathogenic mutations can be corrected with base editors," says Schwank. However, there are still only preclinical tests in animals. With base editors, for example, Schwank's team was able to lower the pathologically increased cholesterol level in mice and macaques by only replacing a DNA component in a gene in the liver. "Efficiency is even lower than that of classic gene scissors, but the potential is there," says Schwank.
The biggest hurdle for in vivo gen therapies remains the transport to the cells. In order to repair a genetic defect, the tools have to get into the corresponding cell via the bloodstream, and this millions of times. The ex-vivo therapies are less affected by the problem: a short circuit briefly absorbs the protein and the associated RNA and are thoroughly examined before applying to the patient. "There are now tried and tested protocols," says Cathomen, who is working on such therapy for HIV patients. In addition to the eye, the inner ear are well suited for Vivo gene therapies. "The liver also works very well because everything that gets into the bloodstream automatically happens to the liver, but if you want to reach other organs, the air becomes thin very quickly."
Voltage and viruses
In order to achieve the liver, scientists use a similar principle as with MRNA vaccination against the coronavirus. Lipidnanoparticles that can only absorb liver cells via certain receptors transport the mRNA information for the editing tools. In contrast to the vaccination, correction of the genetic engineering is the declared goal. Since the mRNA in the cell is dismantled after a few days, the risk of incorrect cuts is reduced. So far, the nanoparticles have only worked in a few cell types.
There is much more experience with viruses than "Gentaxis". Mostly harmless adeno-associated viruses are used. However, many people have already come into contact with related viruses, which is why a large part of these harmless virus transporters are effectively eliminated by the immune system before they reach their destination. "We simply cannot control many organs yet and we still have a long way to go," Cathomen summarizes.
Meanwhile, progress also raises ethical questions. Such therapies can cost up to two million dollars. How should the solidarity financed health systems, like poorer countries, for which some developed therapies would be particularly useful? Sichelzell anemia, for example, is particularly common in Africa south of the Sahara.
And at the latest since the end of 2018, the news has been around the world that a scientist in China has changed embryos with Crispr/Cas9 and this led to the birth of two girls, it is also clear to the public that interventions in the human germination no longer come into the realm Sciecefiction fall.
In embryos, gene defects could probably be corrected more easily, because the gene scissors only have to be introduced into a few cells. However, since sperm and egg cells would also be affected by the correction, it would be heritable. In many countries, including Switzerland and the EU, such interventions are prohibited. In addition to fundamental ethical concerns, there are also tangible medical ones, since the procedures are immature and the long-term consequences are not foreseeable.
And yet the borders are moving: The international commission for the clinical use of genomeditation in germination therapy and also the German Ethics Council, no longer categorical in the future.