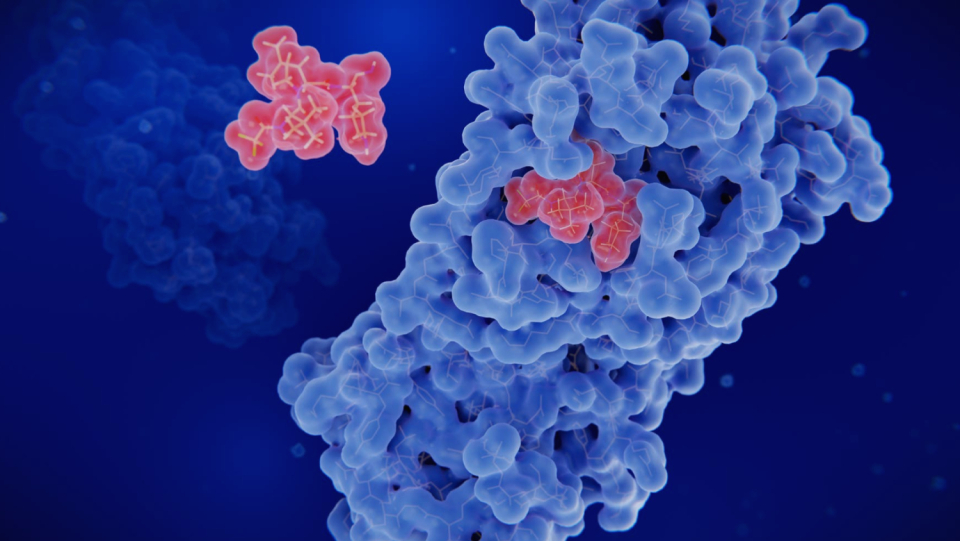
The main protease (Mpro) of Sars-CoV-2, an important target for antiviral drugs, contains a recently discovered switch that reacts to free radicals. A team led by Kai Tittmann identified a so-called NOS bridge there, which forms when the chemical environment is oxidizing enough. In this structure, an oxygen atom enters into chemical bonds with the nitrogen of the amino acid lysine and the sulfur of the amino acid cysteine. As a result, a solid "bridge" is formed between parts of the protein. Such bonds within the protein usually have important effects on function. The discovery could possibly point to new directions for the development of therapies, the working group writes in its publication in "Nature Chemical Biology".
As the team around Tittmann continues, a double bridge is created in the main protease if enough free radicals or oxygen make the environment sufficiently oxidizing. For this purpose, another oxygen atom combines the same nitrogen with another cysteine, so that a pubed bridge is created as a sonos. This suggests that the activity of the protein changes in two stages. As a precise analysis of the molecular structure suggests, the second step is probably the decisive factor. While the first NOS bridge between two amino acids that are already directly opposite and therefore does not change the structure, the second bridge goes to a freely movable part of the protein. This is firmly anchored.
The effects of this switching under different oxidizing conditions are still unclear. Therefore, the findings do not yet suggest a concrete antiviral mechanism of action. However, the structural analysis of Mpro shows that the cysteine bound in the second step interacts without binding with another amino acid in the active site. This region of the protein is crucial for its function; the amino acids there interact with bound proteins that cause them to be cut by the main protease. It is therefore plausible that the formation of the SONOS binding has an influence on the functioning of the viral enzyme, which may be the target of an antiviral drug.