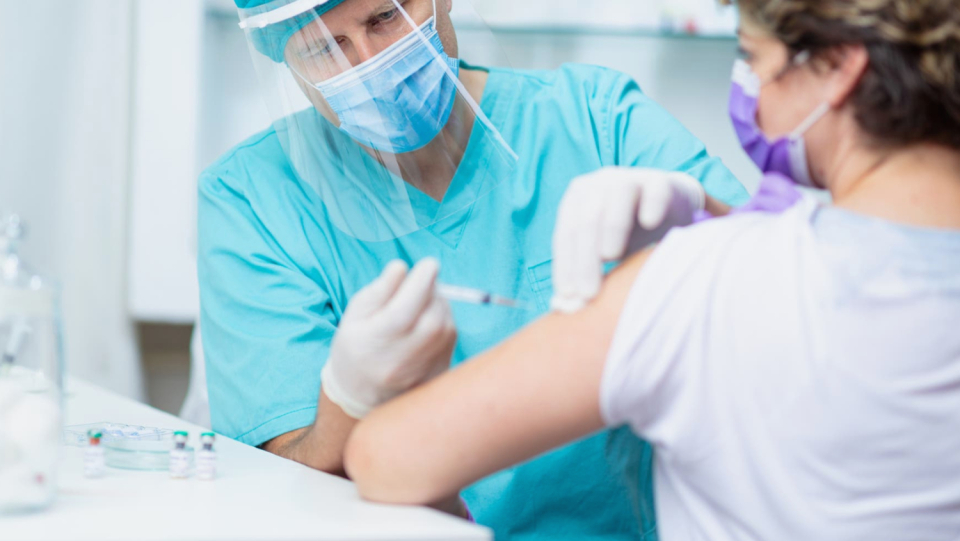
The Standing Committee on Vaccination (STIKO) will probably recommend the vaccines adapted to the Okron variant for the third and fourth vaccine doses. This emerges from the draft vaccination recommendation, which the STIKO has published today. Accordingly, nothing changes in which groups of people the third and fourth vaccination is recommended. However, the Omykron vaccines are to be used preferentially over the previous wild-type vaccinations. The STIKO continues to recommend a third vaccine dose to all persons over the age of 12, and it also advises people over 60 years of age, special risk groups, residents of nursing homes and employees there to have a fourth vaccination.
The EU Commission approved the vaccine from BioNTech and Pfizer, which was developed specifically for the Omikron lines BA.4 and BA.5, the approval was granted in Europe last Tuesday. The European Medicines Agency (EMA) recommends the vaccine as a booster for all people over the age of 12. Previously, there were already vaccines against the Omikron variant BA.the BioNTech and Pfizer have been approved for the No. 1 of Moderna as well as Biontech and Pfizer. All these vaccines are bivalent, that is, they contain two components, one of which creates immunity to the original wild type, the other to omicron.
It is still unclear how much the vaccines actually improve protection against omikron compared to previous vaccines. "All existing vaccines protect very well from serious illness, hospitalization and death," Stiko member Christian Bogdan from the University Hospital Erlangen told the Science Media Center. Immunological data showed a significant improvement in the antibody response against omikron variants by the new vaccines. Because of the short observation period, one could not say whether the vaccination, for example, noticeably reduces symptomatic infections. "What we are actually about is that we can also set ourselves up for any other variants of the Omikron mutant."
Experts expect that the now circulating corona line BA.5 already in a few weeks due to already strongly growing immunoflucht variants such as BA.2.75.2 is replaced. According to neutralization data, the Omikron vaccines also provide only limited protection against such viruses - but probably much better than the pure wild–type vaccination. "How well a vaccination protects against a variant depends on the genetic distance," explains bioinformatician Cornelius Römer from the Biozentrum of the University of Basel. "The Omikron vaccines are also a long way away from lines like BA.2.75.2, but compared to vaccination with the wild type, they are much closer." In this way, they could also reduce the risk that such an immune escape variant undermines the previously very effective protection of vaccinations against serious diseases.
According to many experts, it does not make a big difference whether you get a vaccine against BA.1 or Ba.4/5. This is not only due to new variants, but also because the vaccines are quite similar. While the Spike proteins from Wildtype and BA.1 differ in around 30 positions, there are only three changes between BA..1 and Ba.5. "The interesting thing is that immunization with BA.1 also leads to an improved antibody response against Ba.4/5," says Bogdan. However, so far only the vaccines against BA.1 have been available in humans. So far, the fact that the vaccine against Ba.5 generates the desired immune reaction has only been shown in experiments on mice. "This is certainly a weak point," says Bogdan. "Of course we are not particularly happy on the part of the STIKO."